Design Control 483 Trend
Design Control is a regulatory requirement for the design and development of medical devices and combination (drug/device) products.
In addition, it is a sound engineering practice for design tracking from conception to commercialization and can be used as a project management tool.
There are fundamental elements that constitute the Design Control process. These elements include user needs, design development planning, design input and outputs, design verification and validation, design reviews, and design transfer. However, design control must be linked to other quality system processes and must be utilized throughout the product life-cycle. Design control must be executed using standard procedures and documentation control.
Data analysis of Design Control 483 observations was performed from October 2015 to September 2018 (Reference Figure 1). Based on the data set, the top 80% of 483 observations originated from 21CFR 820.30 subclause (g), (i), (f), (a), and (j).
Figure 1 – Design Control 483 Trend
October 2015 to September 2018
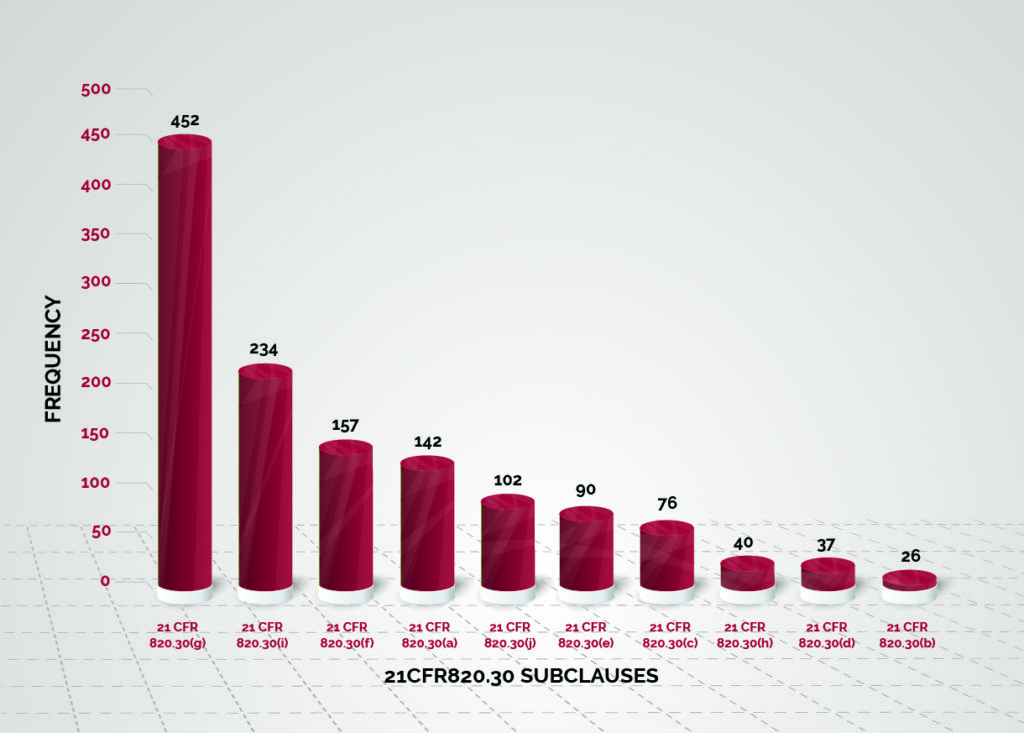
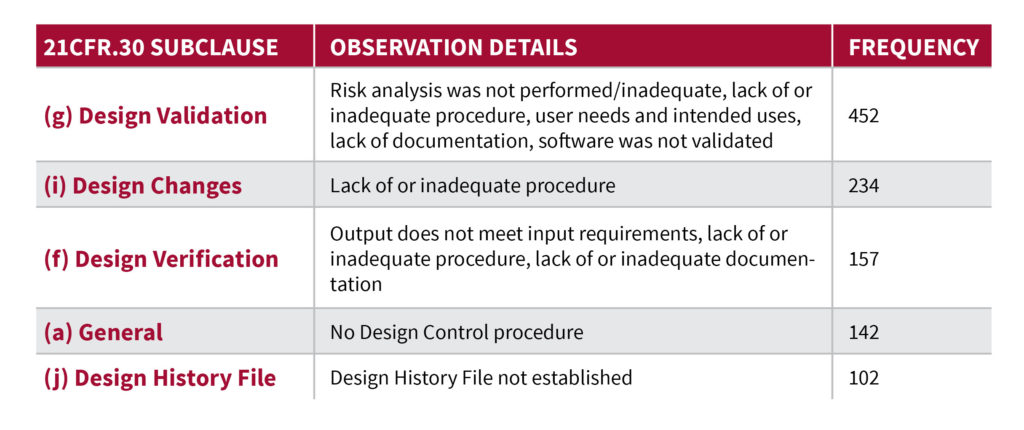
Table 1 provides a summary of the top 80% observations details.
Table 1 – Top 80% of Design Control 483’s
BioTechLogic can provide consultation support for Design Control system remediation, system gap assessment, development and creation of procedures, and design documentation. Please click here to contact us.